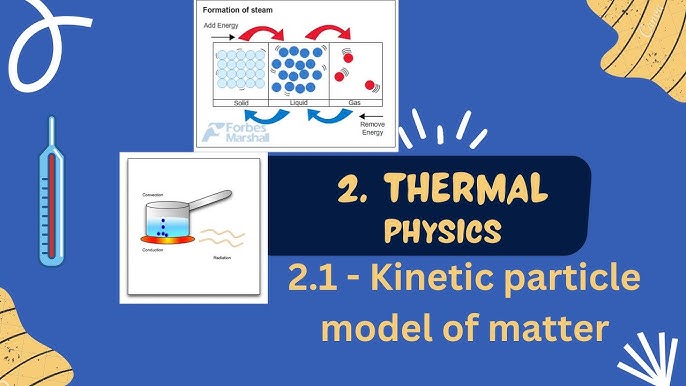
The particle model of matter is a cornerstone concept in thermal physics that explains the behavior and interaction of particles that make up all substances. It provides a simple yet powerful framework to understand phenomena such as heat transfer, changes of state, and particle motion. This article dives deep into the fascinating world of the particle model, its role in thermal physics, and how it connects to our everyday lives.
What Is the Particle Model of Matter?
The particle model of matter is a scientific theory that describes matter as being made up of tiny particles—atoms or molecules—that are constantly in motion. According to this model, the state of matter (solid, liquid, or gas) and its properties are determined by how these particles are arranged and how they interact with each other.
In this model, particles are too small to see directly, but they behave in predictable ways based on temperature and energy. The particle model assumes that:
- All matter is made of particles.
- Particles are always in motion.
- The spaces between particles vary depending on the state of matter.
- The energy of the particles influences their movement and arrangement.
This simple yet powerful framework helps us understand why solids are rigid, liquids flow, and gases expand to fill their containers. It also explains thermal physics processes like conduction, convection, and changes of state.
Why Do Particles Move Faster in Heat?
One of the most fascinating aspects of the particle model of matter is how it relates to heat. Heat is a form of energy, and when particles absorb heat energy, they gain kinetic energy. This increase in kinetic energy causes the particles to vibrate or move faster.
For example, when you heat a pot of water, the particles in the water absorb energy and move more quickly. As a result, the water molecules spread apart, and eventually, the liquid boils and turns into steam (a gas). This principle explains why heating substances often leads to changes in state and why heat transfer is essential in thermal physics.
The relationship between heat and particle motion is central to understanding phenomena like thermal expansion, conduction, and even temperature. Simply put, the more energy particles have, the faster they move and the more likely they are to overcome the forces holding them together.
How Do Particles Behave in Solids, Liquids, and Gases?
The behavior of particles varies significantly in solids, liquids, and gases, which explains the unique properties of these states of matter. Let’s explore each state in detail.
Particles in Solids
In solids, particles are closely packed together in a fixed is particle model of matter thermal physics. They vibrate in place but do not move freely. This tight packing and minimal movement explain why solids have a definite shape and volume. The forces between the particles in solids are strong, making them rigid and resistant to compression.
For instance, in a block of ice, the water molecules are arranged in a crystalline structure that holds the solid together. Even though the particles vibrate, they stay in their fixed positions unless energy is added (e.g., by heating).
Particles in Liquids
In liquids, particles are still close together but not in a fixed arrangement. They can move and slide past one another, which is why liquids can flow and take the shape of their container. The forces between particles in a liquid are weaker than in a solid, allowing for more movement while still maintaining a definite volume.
For example, when you pour water into a glass, the water takes the shape of the glass because the particles are free to move. However, the liquid does not expand to fill the container like a gas would.
Particles in Gases
In gases, particles are far apart and move freely in all directions. The forces between the particles are extremely weak, and the particles have high kinetic energy. This is why gases expand to fill their containers and have neither a definite shape nor volume.
For instance, the air we breathe is a mixture of gas particles moving rapidly and colliding with one another. These collisions create pressure, which is a key concept in thermal physics.
What Happens When Matter Changes State?
Changes in state—such as melting, freezing, boiling, or condensing—occur when particles gain or lose energy. These transitions are governed by the particle model of matter and are a direct result of changes in particle motion and arrangement.
For example, when a solid like ice is heated, its particles absorb energy and begin to vibrate more vigorously. At a certain point, the particles gain enough energy to overcome their fixed positions and slide past one another, turning the ice into liquid water. Similarly, when liquid water is heated further, the particles move even faster until they escape into the air as gas (steam).
Conversely, when energy is removed from a substance, particles slow down and come closer together, leading to processes like freezing or condensation. These changes of state demonstrate how the particle model connects energy, temperature, and matter.
Why Is the Particle Model Important in Thermal Physics?
The particle model is fundamental to thermal physics because it provides a clear explanation of how heat and energy affect matter. It allows scientists and engineers to predict and manipulate the behavior of substances under different conditions.
For instance, the particle model helps explain:
- Heat Transfer: How energy moves from hot objects to cooler ones through conduction, convection, and radiation.
- Thermal Expansion: Why materials expand when heated and contract when cooled.
- Phase Changes: The conditions under which matter changes state, such as boiling or freezing points.
- Pressure and Volume in Gases: The relationship between particle motion, pressure, and volume in gases, described by laws like Boyle’s Law and Charles’s Law.
Without the particle model, understanding these complex phenomena would be nearly impossible.
Fun Facts About the Particle Model of Matter!
Can You See Particles?
No, particles like atoms and molecules are far too small to see with the naked eye. However, scientists use powerful microscopes, such as scanning tunneling microscopes (STMs), to observe particles indirectly. These tools have given us incredible insights into the structure and behavior of matter at the microscopic level.
What About Super Cold Temperatures?
At extremely low temperatures, particles move so slowly that matter can enter a unique state known as a Bose-Einstein Condensate (BEC). In this state, particles behave like a single entity and exhibit strange quantum properties. This state was first observed in the lab in 1995 and is a fascinating area of study in modern physics.
What’s Absolute Zero?
Absolute zero is the lowest possible temperature, where particles have minimal motion and no kinetic energy. It’s equivalent to -273.15°C or 0 Kelvin. While absolute zero cannot be achieved in practice, scientists have come very close to reaching this limit in laboratory experiments.
How Does the Particle Model Connect to Everyday Life?
The particle model isn’t just a theoretical concept—it has practical applications in our daily lives. From cooking food to understanding weather patterns, the particle model explains countless everyday phenomena.
For example, when you boil water for tea, the particle model describes how heat energy makes water molecules move faster until they escape as steam. Similarly, refrigerators use the principles of particle motion and energy transfer to keep food cold by slowing down particle movement. Even technologies like air conditioning and car engines rely on thermal physics concepts derived from the particle model.
The Bottom Line
The particle model of matter is a foundational concept in thermal physics that helps us understand the behavior of solids, liquids, and gases. By describing matter as tiny particles in constant motion, this model explains everything from heat transfer to changes of state. Its relevance extends far beyond science classrooms, shaping technologies and processes that impact our everyday lives.
Understanding the particle model not only deepens our appreciation for the physical world but also empowers us to harness its principles for innovation and problem-solving. So, whether you’re a student learning about thermal physics or someone curious about the science behind everyday phenomena, the particle model offers a simple yet profound way to explore the mysteries of matter.